When characterizing solid materials, specific surface area is often one of the first properties scientists look to determine. It influences everything from adsorption capacity and reactivity to dissolution and formulation behavior.
Traditionally, researchers have relied on volumetric gas adsorption techniques to calculate surface area, but these methods can be slow, indirect, and sensitive to experimental conditions.
Inverse Gas Chromatography (iGC) offers a more dynamic, reproducible alternative. By measuring how gases move through and interact with a packed column of material—rather than how they accumulate under static pressure—iGC reveals surface properties with precision, speed, and flexibility across powders, fibers, and films.
Volumetric Methods
A quantitative method for determining the concentration of a substance in a solution by measuring the volume of another solution of known concentration that reacts with it
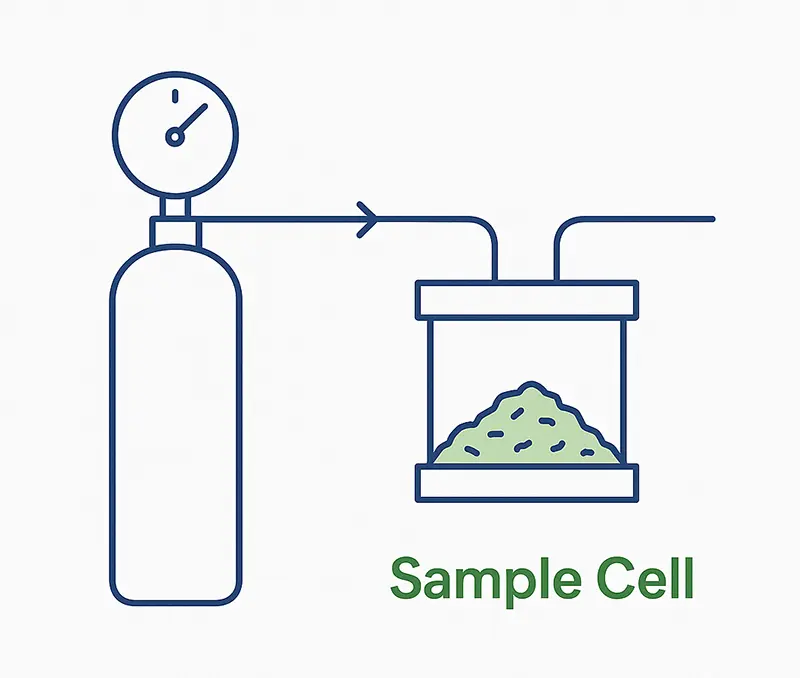
Volumetric Setup:
Measuring Pressure to Infer Adsorption
Volumetric gas adsorption relies on a sealed chamber filled with an inert gas and a powder sample. As the gas is introduced, the system records how the internal pressure drops when molecules adhere to the sample surface. By repeating this process at different pressures, a characteristic adsorption curve is constructed.
This setup, while conceptually simple, measures surface interaction indirectly through pressure change rather than molecular behavior. It requires precise calibration, temperature stability, and long equilibration times—factors that can introduce variability and limit throughput.
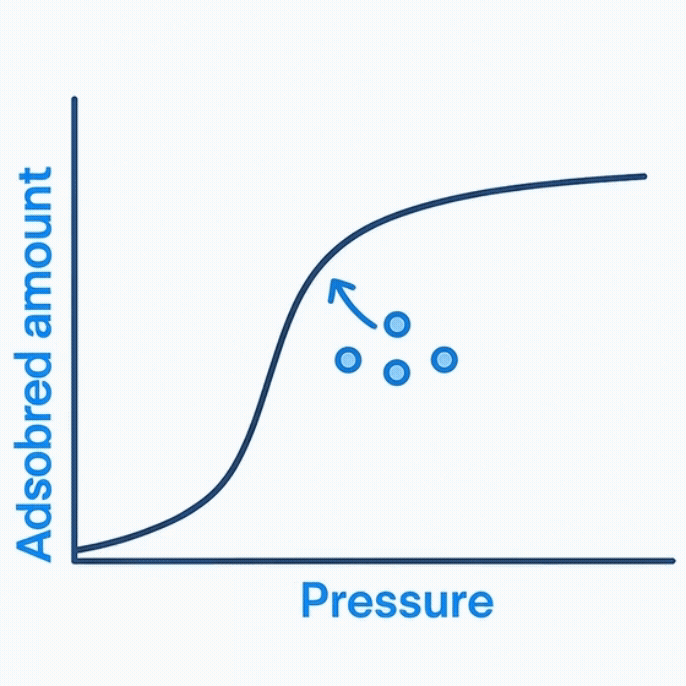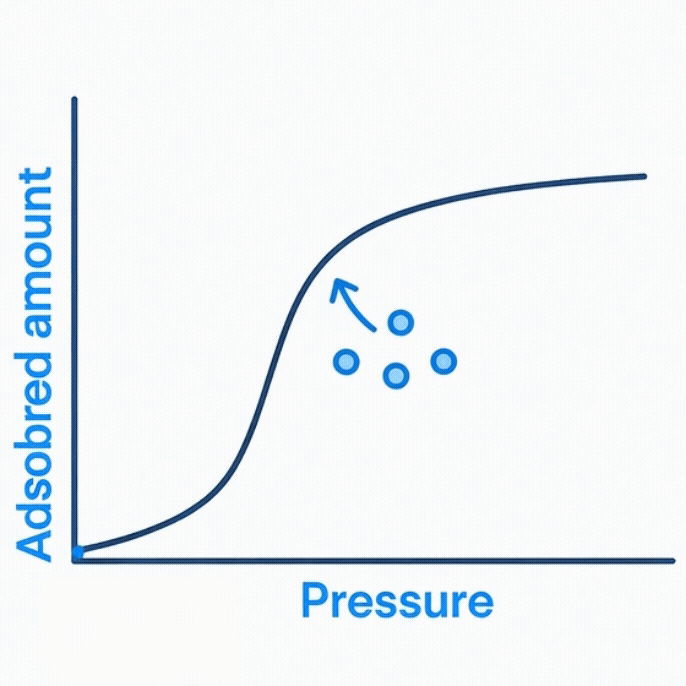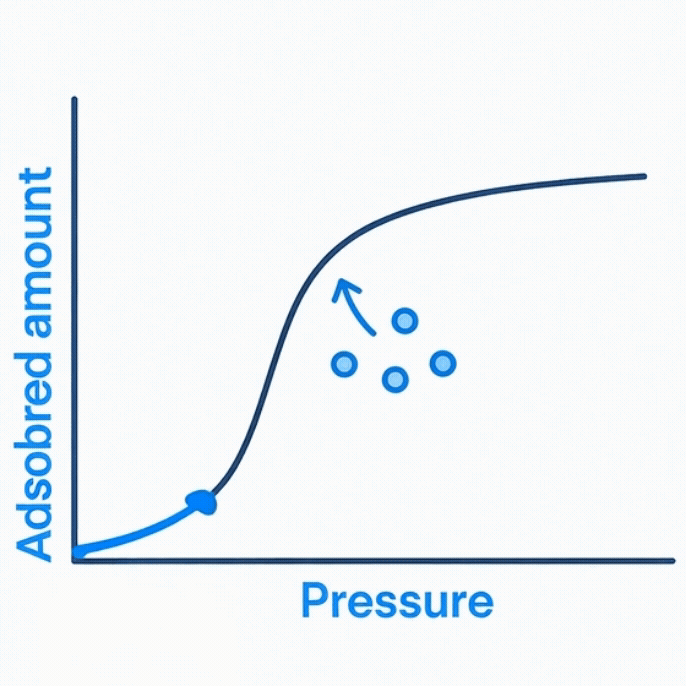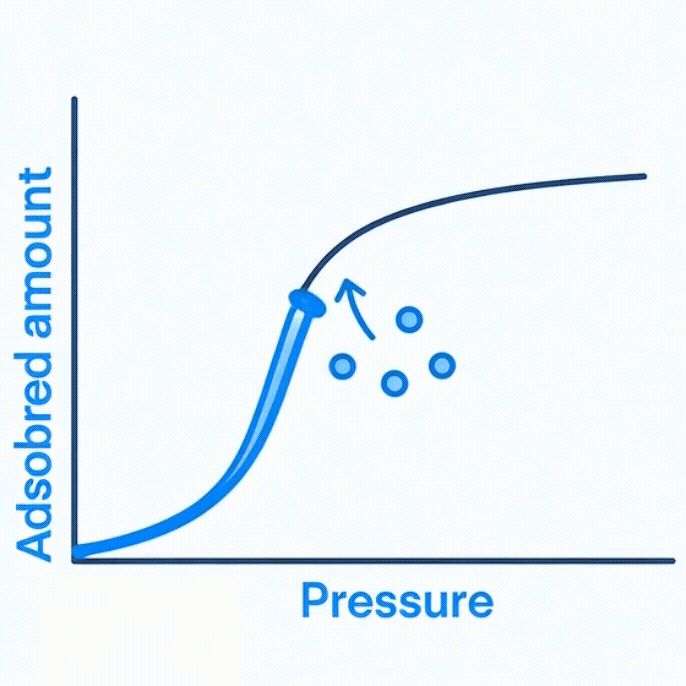
Analyzing Data:
Brunauer–Emmett–Teller (BET) Equation
Once adsorption and desorption cycles are complete, the collected pressure data are fitted to the Brunauer–Emmett–Teller (BET) equation. The linear portion of the curve allows determination of the monolayer capacity, from which the specific surface area is calculated.
However, the accuracy of BET surface area depends heavily on correct model fitting and the assumed shape of the isotherm. Small experimental deviations—moisture contamination, leaks, or temperature drift—can cause significant discrepancies in surface area values between laboratories.
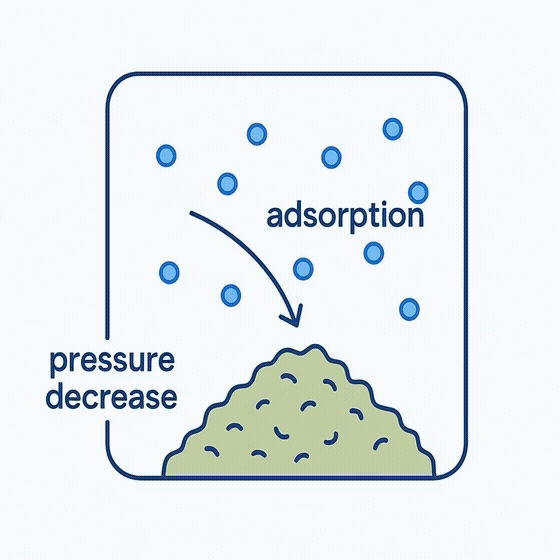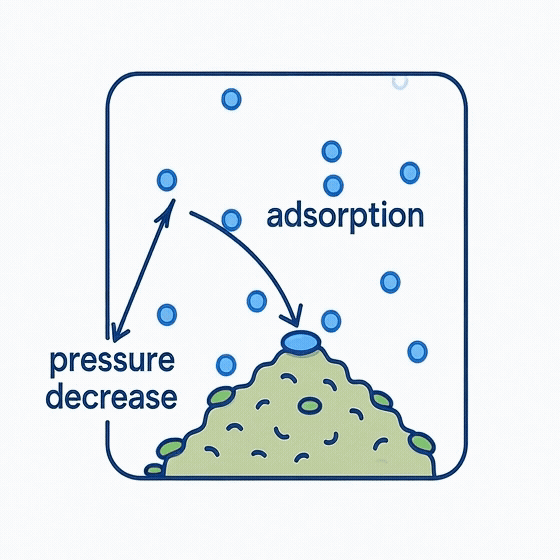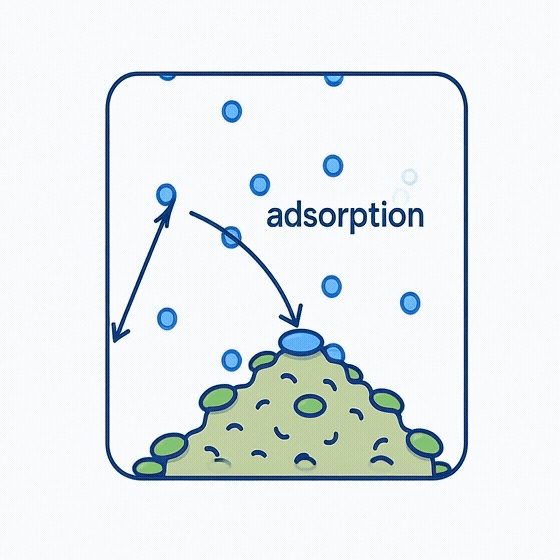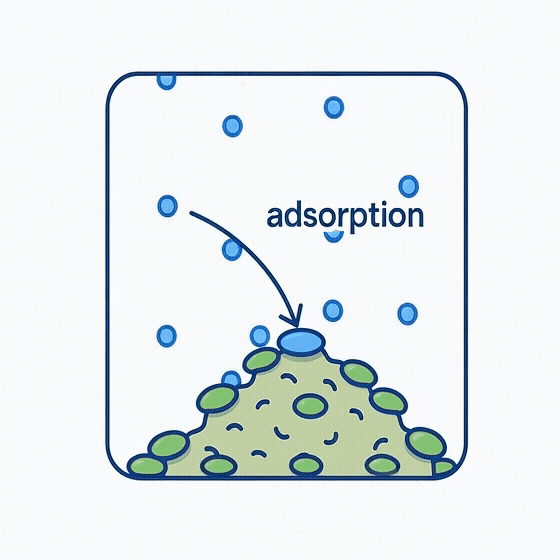
Volumetric Adsorption:
From Pressure to Surface Coverage
Inside the sealed chamber, gas molecules gradually find and attach to available surface sites on the sample. As more molecules are adsorbed, fewer remain in the gas phase, leading to a measurable decrease in pressure.
Although this allows calculation of total adsorbed volume, it provides little information about how molecules interact with different surface regions, and assumes idealized adsorption behavior—conditions that often don’t hold true for complex or heterogeneous materials.
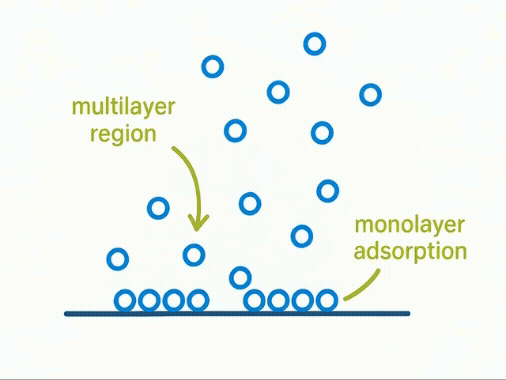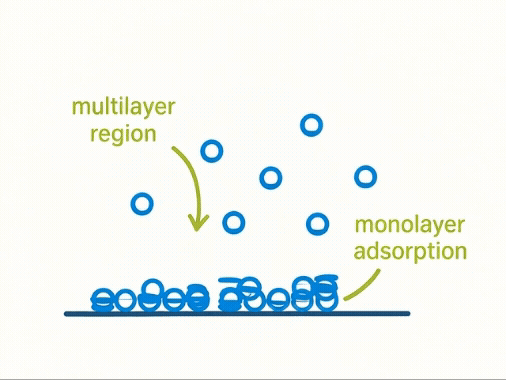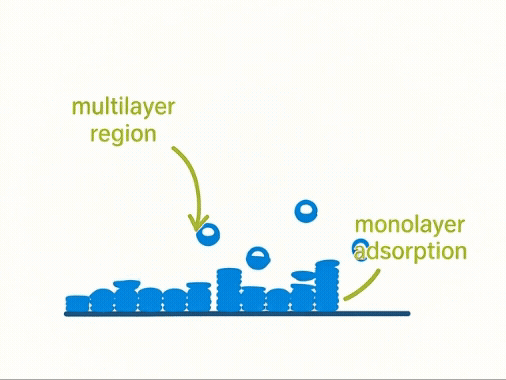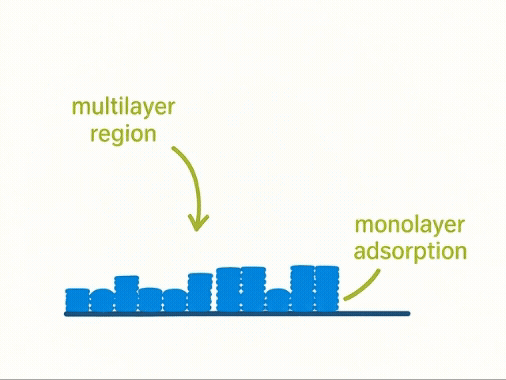
Taking a Closer Look:
Layers of Adsorption
At a molecular level, volumetric adsorption assumes a progressive build-up of gas molecules forming monolayers and multilayers across a solid surface. The first layer—known as the monolayer—is key to calculating surface area using the BET model.
Yet, this assumption simplifies complex physical reality. Not all surfaces adsorb uniformly, and multilayer formation often begins before the monolayer is complete. As a result, the model may over- or under-estimate true surface area for real-world materials.
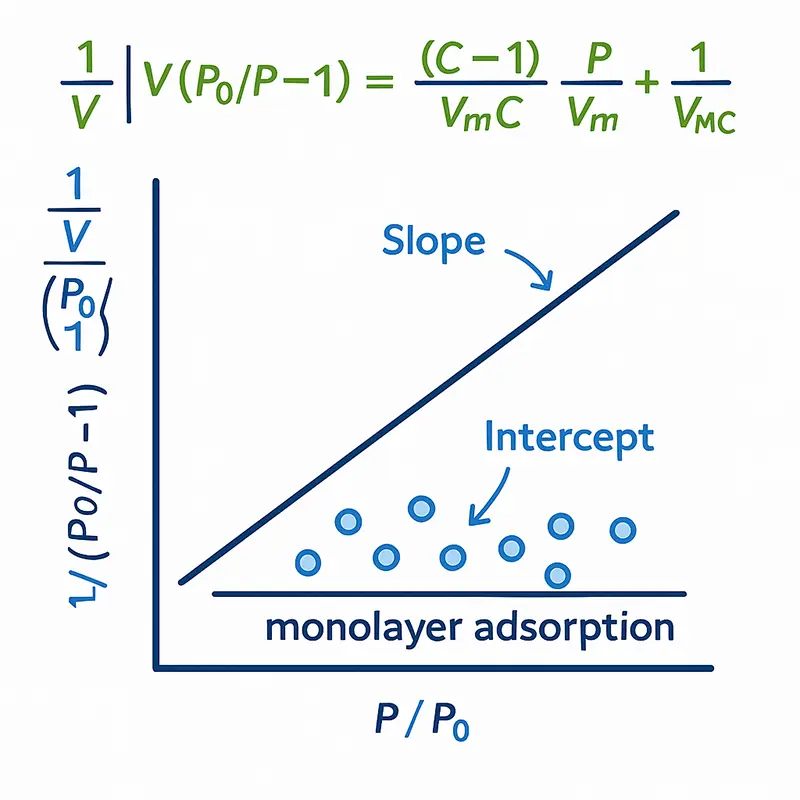
Data Interpretation:
Indirect Assumptions
The BET equation transforms measured pressure data into a linear relationship, allowing scientists to extract the monolayer volume and calculate surface area. It’s a foundational approach, but its reliability depends on correct selection of the “BET range,” an often subjective step.
This mathematical dependency on fitting and assumptions is a key limitation: the surface area result is not measured directly, but inferred. iGC bypasses this problem by observing molecular retention directly from chromatographic data.
Chromatographic Methods
Techniques for separating a mixture into its individual components by distributing them between a mobile phase and a stationary phase. The components move at different speeds based on their affinity for each phase, allowing for separation, analysis, and purification.

Inverse Gas Chromatography Set up:
A Dynamic Flow Approach
In contrast to the static nature of volumetric systems, iGC employs a dynamic, continuous flow of gas through a column packed with the sample material. The technique measures how long it takes different probe molecules to pass through, directly linking flow behavior to surface interaction.
This simple yet elegant setup eliminates the need for pressure measurement or vacuum calibration. Instead, it records the retention time of gas molecules—an intrinsic signal of surface area and surface energy—using standard chromatographic detectors.
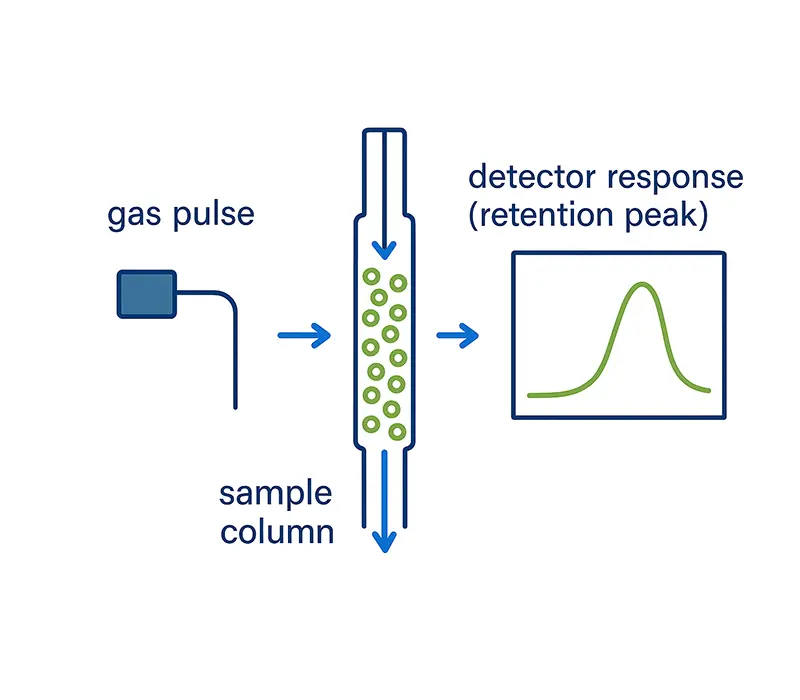
iGC in Action:
Pulse, Flow, Direct
During analysis, a small pulse of vapor is injected into the carrier gas stream and travels through the column. Molecules interact with the surface—some weakly, others more strongly—before reaching the detector, producing a sharp, quantifiable retention peak.
Each peak’s shape and delay encode valuable information about the surface: how much area is available for adsorption, how heterogeneous the sites are, and how strongly molecules are bound. Unlike volumetric methods, iGC observes surface behavior in motion, not just at equilibrium.
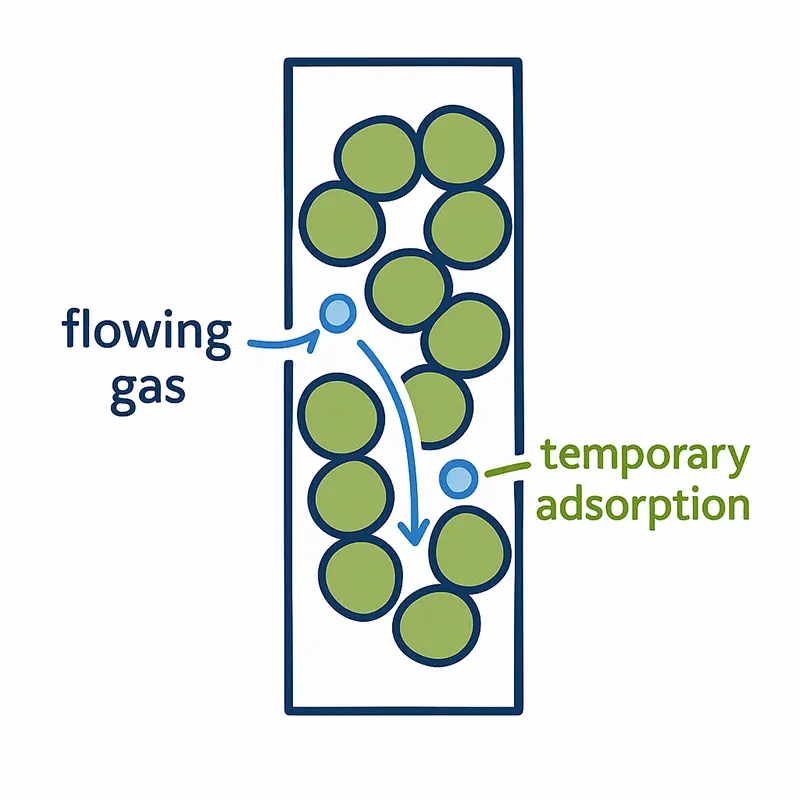
Inside the column:
Molecular Interactions in iGC
Within the iGC column, flowing gas molecules make transient contact with the packed solid particles. Each collision represents a temporary adsorption event that slightly delays the molecule’s passage. The overall retention time reflects the sum of these interactions.
Because iGC measures these dynamic interactions continuously, it captures how surface area and surface energy jointly influence molecular movement—offering richer, more representative data than static adsorption equilibria.
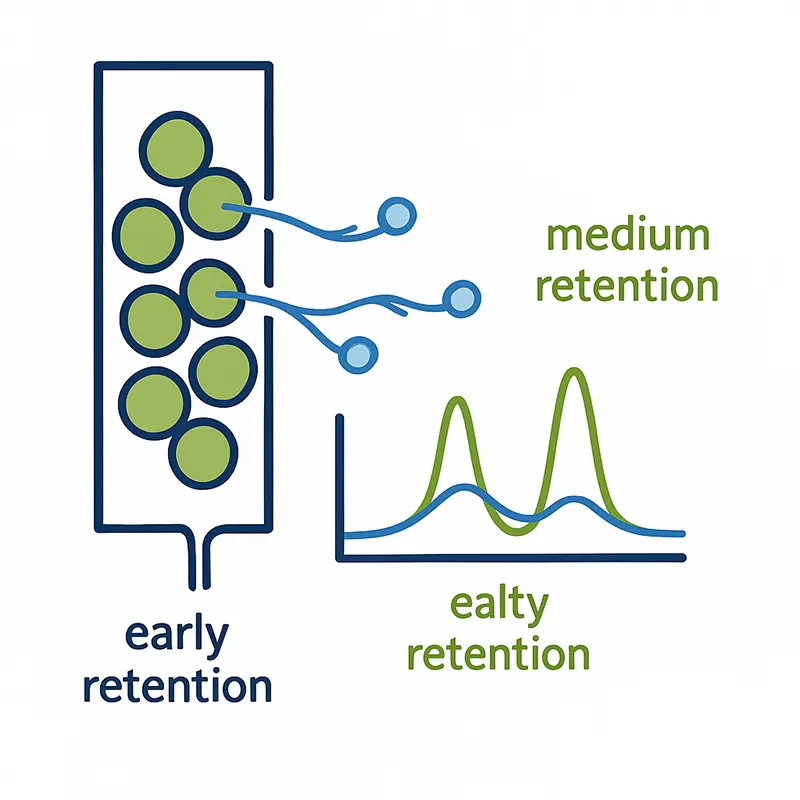
Retention Times:
The Fingerprint of Surface Properties
Different probe molecules, with varying size and polarity, experience different degrees of interaction with the surface. In chromatographic terms, this appears as distinct retention peaks: weaker interactions elute early, stronger interactions later.
By analyzing these retention profiles, iGC can separate the effects of surface area from surface chemistry. This multi-dimensional insight is simply not available in conventional volumetric methods, which measure only bulk adsorption capacity.
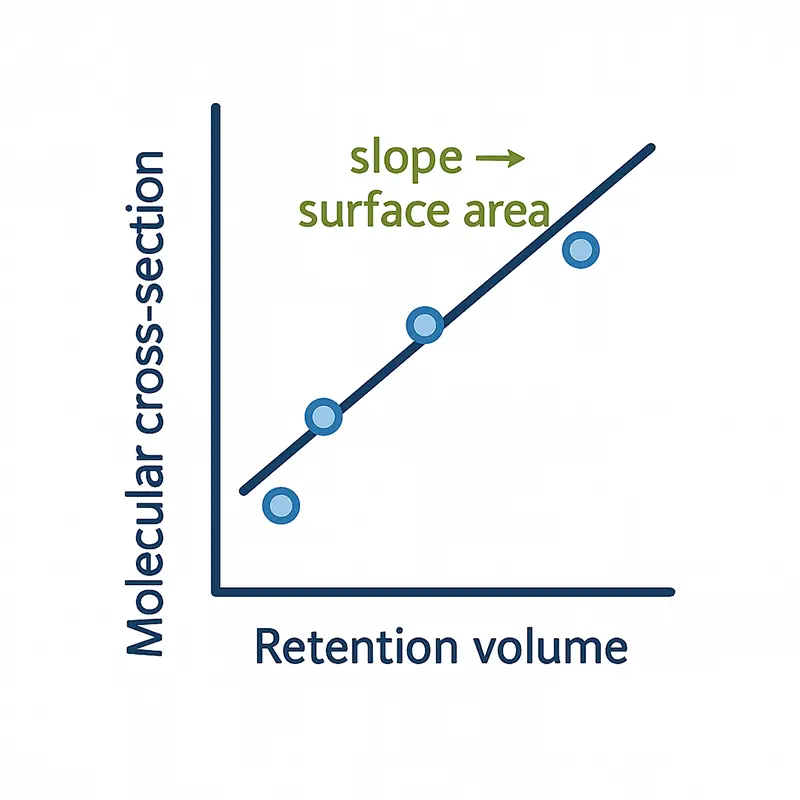
Determining Surface Area:
Interpreting Retention Time Data
From a series of probe gases with known molecular cross-sections, a linear relationship is established between retention volume and molecular size. The slope of this line directly yields the specific surface area—no isotherm fitting, no complex assumptions.
This approach delivers rapid, reproducible measurements even for materials unsuitable for traditional adsorption methods, such as fibers, films, or cohesive powders. It’s surface science simplified through kinetics, not equilibrium.

Volumetric vs. Chromatographic
Comparing Approaches to Surface Area Determination
The determination of surface area has long been a cornerstone of materials characterization, and for decades, volumetric gas adsorption techniques have served as the default approach. However, as materials have grown more diverse—ranging from fine powders and cohesive fibers to thin films and porous composites—the limitations of static adsorption have become increasingly evident. Volumetric systems measure the absence of gas (via pressure change), not the behavior of molecules on real surfaces. This indirect approach, while well-established, often struggles with non-ideal samples, long equilibration times, and model-dependent data interpretation.
Chromatographic techniques, particularly Inverse Gas Chromatography (iGC), reframe the same measurement through a dynamic, flow-based lens. Rather than observing a closed system reach equilibrium, iGC measures how probe molecules travel through and interact with the sample in real time. Each retention event directly reflects surface interactions — offering a more immediate and kinetic view of the sample’s accessible surface area. The technique requires less sample preparation, avoids vacuum or pressure corrections, and delivers results in a fraction of the time. More importantly, iGC can characterize materials that are challenging or impossible to measure using volumetric methods, such as low surface area powders, fibrous samples, or coatings on films.
In practical terms, iGC eliminates many of the assumptions that limit the reliability of volumetric approaches. It does not rely on fitting models like BET or on selecting arbitrary “linear regions” of isotherms. Instead, it extracts quantitative surface parameters directly from retention data, producing reproducible results that correlate strongly across laboratories. Its continuous-flow nature also enhances sensitivity, enabling detection of subtle changes in surface area or chemistry caused by treatments, coatings, or humidity. For modern material development—where speed, reproducibility, and sample diversity are key—chromatographic techniques offer not only a more efficient measurement but also a deeper understanding of surface behavior.
Key Features
How do these techniques compare?
Measurement Principle
Volumetric Adsorption
Brunauer, Emmett, and Teller (BET) method
Static adsorption: monitors pressure drop as gas adsorbs
Chromatographic
Inverse Gas Chromatography
Dynamic flow: measures retention time of probe gases
Data Type
Indirect (pressure vs. adsorbed volume)
Direct (molecular retention and interaction)
Model Dependency
Requires BET or similar fitting models
Minimal modelling; data derived directly from flow
Sample Requirements
Requires degassing, dry conditions, and stable powder form
Works with powders, fibers, films, and coatings
Analysis Time
Long equilibration; several hours per sample
Fast throughput; often under 30 minutes
Reproducibility
Sensitive to leaks, temperature, and pressure errors
High reproducibility across runs and instruments
Sensitivity
Limited for low surface area or heterogeneous materials
Excellent sensitivity across wide material types
Information Depth
Provides surface area only
Standard chromatographic flow system
Operational Simplicity
Requires expert control of conditions
Simple setup, minimal calibration
Have More Questions?
This is just the tip of the ice berg for Inverse Gas Chromatography. With a range of applications across the industrial materials spectrum, it could be the secret weapon your research is missing. Use the resources below to find out more
Learn more about iGC
Reading our company web page on Inverse Gas Chromatography to get more details on how the technique works.
Ask Prof. Sorption...
Ask our own little helper, Prof. Sorption, for more unique queries. He's been trained up on all things sorption science and iGC, and can't wait to help.

Consult with our science team
Is iGC just what you've been looking for? Book a committment-free consult with our team to see how iGC can benefit your research.

