
Sorption Science Webinars/Workshops Directory
To keep up to date with our upcoming events please visit: https://www.surfacemeasurementsystems.com/learning-center/webinars/
| Responsible | Daniel Villalobos |
|---|---|
| Last Update | 05/02/2026 |
| Completion Time | 1 day 5 hours 35 minutes |
| Members | 254 |

Webinar title: Particle Engineering in Pharmaceutical Solids Processing: Surface Energy Considerations
Topic: This webinar presented by Dr Daryl Williams from Imperial College London reviews the surface energy and surface energy heterogeneity of crystalline solids, methods for the measurement of surface energy, effects of milling on powder surface energy, adhesion and cohesion on powder mixtures, crystal habits and surface energy, surface energy and powder granulation processes.
For more information visit our website:
www.surfacemeasurementsystems.com
LinkedIn: https://www.linkedin.com/company/surface-measurement-systems
Twitter: https://twitter.com/surfacemsystems
Porous materials
View all

With the release of the DVS Carbon, researchers now have a powerful, purpose-built tool for characterizing materials for Carbon Capture, Utilization, and Storage (CCUS) applications. With unique measurement and engineering capabilities, we are excited to demonstrate what this new instrument can do! In this webinar, Product Manager Dr. Paul Iacomi takes the audience through the unique features of this innovative instrument, having a closer look at the DVS Carbon’s capabilities in action. Case studies on the instrument’s applications, including Direct Air Capture and Post-combustion Capture, show how to utilize the DVS Carbon in your own research.

This workshop was organized with Universität Magdeburg and explores advanced and innovative sorption techniques for analyzing catalysts, adsorbents, and functional materials for heat pumps. Delivered by the world’s leading sorption instrumentation specialists, Surface Measurement Systems, the session dives into the advanced gravimetric sorption technique, Dynamic Vapor Sorption (DVS). This webinar provides insight into applications for studying zeolites, binary co-sorption of MOFs, VOC capture, silica gels and adsorption heat pumps.
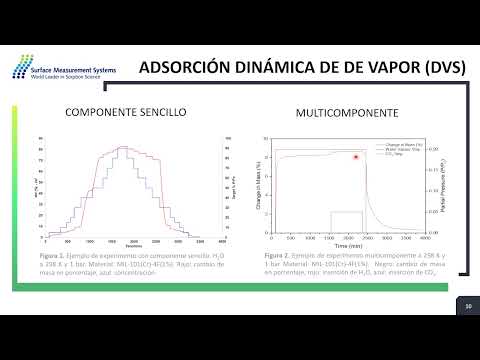
Spanish Seminario : Métodos experimentales para la captura de CO2 en presencia de vapor de agua

This session will cover Metal Organic Frameworks (MOFs) that can be used in a range of adsorption applications, such as gas storage, carbon capture, separations, catalytic transformation, and drug delivery. In addition, they can be also used for thermochemical energy storage applications, whereby heating and cooling technologies use thermo-adsorptive effects.
The combination of hierarchical pore structure control of these materials and the selection of appropriate adsorbent enables entry and adsorption of small molecules on internal surfaces. Such processes are typically controlled by physisorption mechanisms governed by molecular size, polarity, and chemical nature of the sorbent surfaces. In some cases, specific chemical interactions can give rise to more strongly bound chemisorbed species which are in essence part in the design of heterogeneous catalysts
Water sorption, Organic sorption and Gas adsorption measurements on MOFs can provide valuable information about the effect of probe molecules on materials stability, kinetics, adsorption capacity, and energy requirements for the regeneration of adsorbents.
This webinar highlights an innovative experimental method for determining gas and vapor adsorption and co-adsorption isotherms on MOFs using a novel dynamic vacuum flow configuration in a broad temperature range.

Dr. Ilich A. Ibarra
Associate Professor
Instituto de Investigaciones en Materiales, UNAM,
Circuito exterior Ciudad Universitaria C.P. 04510, Ciudad de México
Abstract:
Hydrogen sulphide (H2S) is a harmful chemical present in natural gas, biogas and emitted by different chemical industries, e.g., oil desulfurization process at oil refineries. H2S is considered as a major air pollutant due to its negative environmental impact, mainly associated with acid rain, and to high toxicity to humans leading to severe nervous system illnesses.
On the other hand, sulphur dioxide (SO2) considered as one of the most hazardous chemicals is a colourless, non-flammable gas with a strong odour. SO2 provokes severe health issues including alterations of the respiratory system (e.g., broncho-constriction in lung function). Typically, an exposure to only 1.5 ppm of SO2 for a few minutes can cause a temporary incapability to breath normally. Moreover, this
chemical is highly soluble in water and forms sulphurous acid further converted to sulfuric acid, the main component of acid rain which can damage plants, accelerate the corrosion of metals and attack limestone, marble, mortar, etc. The harmful impact of this pollutant present in the atmosphere is also catastrophic in terms of global warming, ozone depletion and climate change.
Metal-Organic Frameworks (MOF) have been envisaged for the capture of H2S and SO2 however, some of them, with the main disadvantage of showing poor chemical stability. Thus, in this talk we present two MOF materials highly chemically-stable to H2S and SO2: MIL-53(Al)-TDC7 and MFM-300(Sc), respectively.
MIL-53(Al)-TDC is demonstrated to exhibit one of the highest H2S capture (18.5 mmol g-1 at 298 K and 1 bar) ever reported for a MOF, to the best of our knowledge, along with the retention of its crystalline structure after multiple H2S adsorption/desorption cycles and an excellent regeneration at relatively low temperature. MFM-300(Sc) is demonstrated to exhibit a SO2 uptake of 9.4 mmol g-1 at 298 K and 1 bar significantly higher compared to its Al- and In-analogues, along with the retention of this level of performance after multiple SO2 adsorption/desorption cycles owing to the high stability of its crystalline structure. Advanced experimental and computational tools have been further coupled to gain insight into
the molecular mechanisms responsible for the adsorption of H2S and SO2.

Abstract:
Wavy nickel nanowires (NiNWs) were immobilized on mesoporous silica (SiO2) aerogels by the sol−gel method. The catalytic activity of pure NiNWs and NiNW−SiO2 aerogel composites toward the CO2 hydration reaction (CHR) when they are in water was measured. Dynamic Vapor Sorption (DVS Vacuum) analysis was performed at levels of 50% CO2 and 50% H2O vapor for SiO2 aerogels, immobilized nickel nanoparticles (NiNPs) on silica aerogel and NiNW−SiO2 aerogel composites, in order to determine catalytic activity for CHR in the gaseous phase. The results from DVS Vacuum analysis (gaseous phase) and CHR (aqueous phase) showed that NiNW−SiO2 aerogel composites are good heterogeneous catalysts for CHR in both gaseous and aqueous phases but they are less active than NiNP−SiO2 aerogel composites

Presenter: Dr. Vladimir Martis
Abstract
Crystalline solids with controllable structures possess tailored porosity and large surface areas. This is particularly attractive for gas storage and separation applications. Physisorption of gases is a technique applied for the characterization of porous solids, such as zeolites and metal-organic frameworks (MOFs). Gravimetric vapor sorption and gas adsorption techniques measure promising functionalities such as removal of carbon dioxide (CO2) from the atmosphere or stability of sorbents in humid environments. Currently, both techniques are routinely applied for characterization of porous solids to explore adsorption capacities and porosity. However, only few studies focus on the pre-experimental conditions for the determination of gas/vapor sorption isotherms. Details of outgassing conditions, despite their importance, are often lacking in research publications. Outgassing at low temperatures of thermally stable material provide an incomplete cleaning of the porous surface. As a result, the ability of sorbents to store CO2 or water molecules is underestimated based on adsorption data. Contrary, outgassing of temperature sensitive sorbent at elevated temperatures can cause irreversible structural changes which will have profound effects on the adsorption capacities. The impact of water adsorption on the structure was isolated by introducing partial pressures of water under vacuum. Such measurements provided a true water adsorption isotherm without unnecessary interference from a carrier gas. CO2 adsorption data were measured from low pressures up to 1bar. CO2 adsorption in the presence of water on the sorbent was collected in a system under vacuum. The results show that the performance of the sorbent can be significantly modified depending on outgassing conditions.